The Number of Electrons in the Outer Shells of the Members of the Sodium Family
3.i: Electron Configurations
- Folio ID
- 119829
Skills to Develop
- Derive the predicted ground-state electron configurations of atoms
- Identify and explain exceptions to predicted electron configurations for atoms and ions
- Predict the charge of common metallic and nonmetallic elements, and write their electron configurations
- Relate electron configurations to element classifications in the periodic tabular array
Having introduced the basics of atomic structure and quantum mechanics, we tin use our understanding of breakthrough numbers to determine how atomic orbitals relate to ane some other. This allows us to determine which orbitals are occupied past electrons in each atom. The specific arrangement of electrons in orbitals of an atom determines many of the chemic properties of that atom.
Orbital Energies and Atomic Construction
The free energy of atomic orbitals increases as the chief quantum number, \(n\), increases. In whatsoever atom with two or more than electrons, the repulsion betwixt the electrons makes energies of subshells with dissimilar values of \(l\) differ so that the energy of the orbitals increases inside a vanquish in the club southward < p < d < f. Figure \(\PageIndex{1}\) depicts how these two trends in increasing energy relate. The anesouthward orbital at the lesser of the diagram is the orbital with electrons of lowest energy. The energy increases equally nosotros move up to the iidue south so 2p, 3south, and 3p orbitals, showing that the increasing north value has more influence on energy than the increasing l value for small atoms. However, this pattern does non hold for larger atoms. The 3d orbital is higher in energy than the 4s orbital. Such overlaps continue to occur frequently as we move upwards the nautical chart.
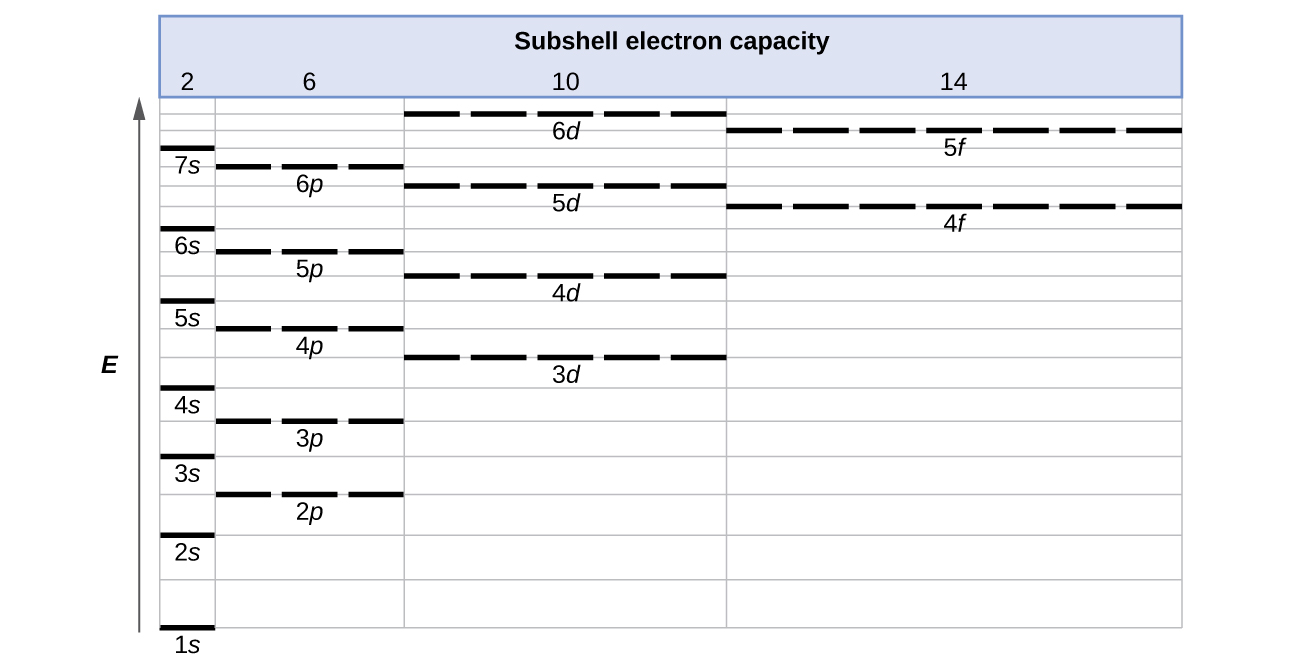
Figure \(\PageIndex{1}\) : Generalized energy-level diagram for diminutive orbitals in an atom with two or more electrons (not to scale).
Electrons in successive atoms on the periodic table tend to fill low-energy orbitals first. Thus, many students find it confusing that, for case, the 5p orbitals fill immediately after the 4d, and immediately before the 6s. The filling social club is based on observed experimental results, and has been confirmed by theoretical calculations. As the primary quantum number, n, increases, the size of the orbital increases and the electrons spend more time further from the nucleus. Thus, the attraction to the nucleus is weaker and the free energy associated with the orbital is higher (less stabilized). But this is not the only effect we have to accept into business relationship. Inside each crush, equally the value of l increases, the electrons are less penetrating (meaning at that place is less electron density found close to the nucleus), in the order s > p > d > f. Electrons that are closer to the nucleus slightly repel electrons that are farther out, offsetting the more ascendant electron–nucleus attractions slightly (recall that all electrons have −i charges, merely nuclei have +Z charges). This miracle is called shielding and will be discussed in more item in the next section. Electrons in orbitals that experience more than shielding are less stabilized and thus college in free energy. For small orbitals (ones through 3p), the increase in free energy due to n is more pregnant than the increment due to fifty; withal, for larger orbitals the 2 trends are comparable and cannot be merely predicted. We will talk over methods for remembering the observed order.
The arrangement of electrons in the orbitals of an atom is chosen the electron configuration of the cantlet. We describe an electron configuration with a symbol that contains three pieces of data ( Effigy \(\PageIndex{two}\)):
- The number of the master breakthrough shell, north,
- The alphabetic character that designates the orbital type (the subshell, l), and
- A superscript number that designates the number of electrons in that item subshell.
For example, the notation 2p 4 (read "2–p–4") indicates four electrons in a p subshell (l = ane) with a principal breakthrough number (northward) of ii. The notation 3d 8 (read "iii–d–eight") indicates viii electrons in the d subshell (i.e., l = 2) of the principal beat for which n = 3.
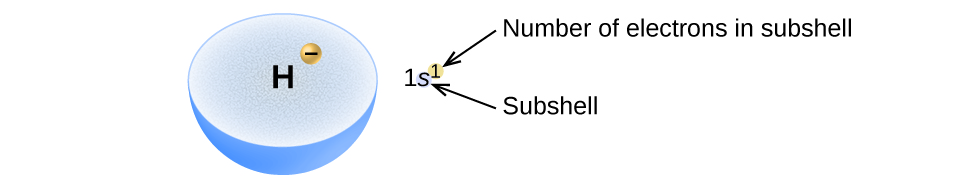
Figure \(\PageIndex{2}\) : The diagram of an electron configuration specifies the subshell (due north and l value, with letter symbol) and superscript number of electrons.
The Aufbau Principle
To determine the electron configuration for whatever particular atom, we tin "build" the structures in the order of atomic numbers. Beginning with hydrogen, and continuing across the periods of the periodic table, we add one proton at a time to the nucleus and one electron to the proper subshell until we have described the electron configurations of all the elements. This procedure is called the Aufbau principle, from the German word Aufbau ("to build up"). Each added electron occupies the subshell of lowest energy available (in the guild shown in Figure \(\PageIndex{3}\)), subject to the limitations imposed by the allowed breakthrough numbers according to the Pauli exclusion principle. Electrons enter higher-energy subshells only after lower-energy subshells have been filled to capacity. Effigy \(\PageIndex{3}\) illustrates the traditional way to remember the filling order for atomic orbitals.
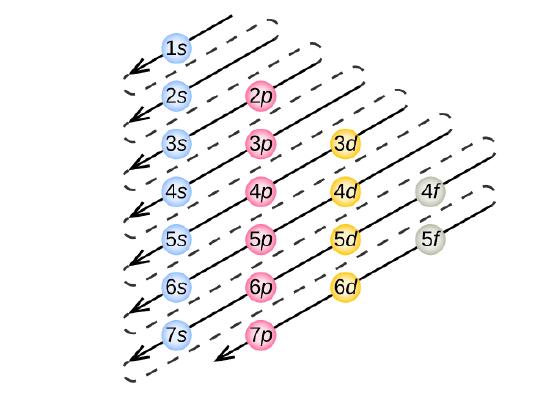
Figure \(\PageIndex{3}\) : The arrow leads through each subshell in the appropriate filling order for electron configurations. This chart is straightforward to construct. Only make a column for all the s orbitals with each n crush on a separate row. Repeat for p, d, and f. Be sure to only include orbitals allowed by the quantum numbers (no 1p or 2d, and then forth). Finally, draw diagonal lines from summit to bottom as shown.
Since the arrangement of the periodic tabular array is based on the electron configurations, Figure \(\PageIndex{4}\) provides an alternative method for determining the electron configuration. The filling gild only begins at hydrogen and includes each subshell every bit you go along in increasing Z order. For example, after filling the iiip cake upward to Ar, nosotros see the orbital will be 4s (K, Ca), followed by the iiid orbitals.
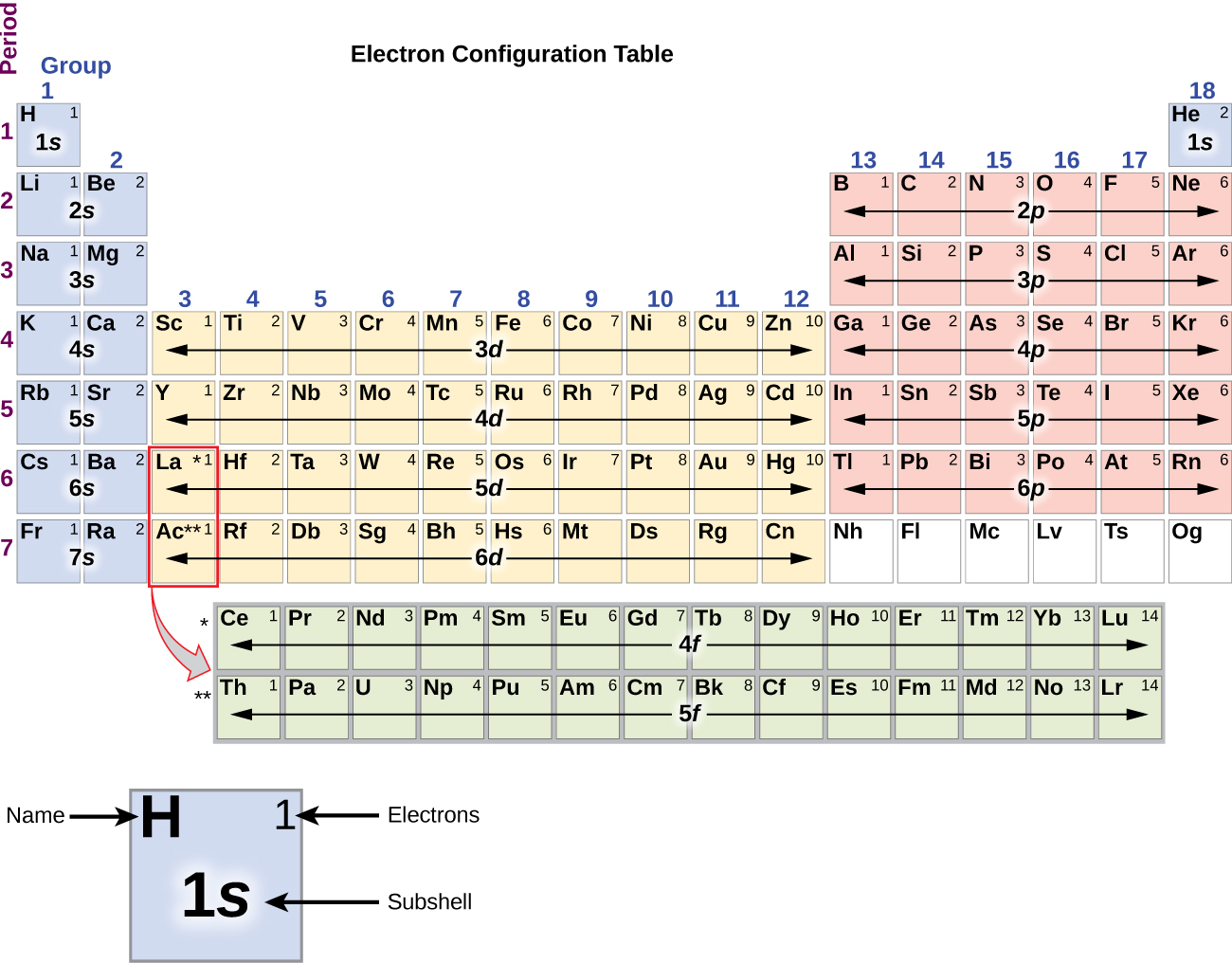
Figure \(\PageIndex{4}\): This periodic tabular array shows the electron configuration for each subshell. Past "edifice upwards" from hydrogen, this table can be used to determine the electron configuration for whatsoever cantlet on the periodic tabular array.
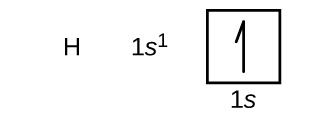
We will now construct the footing-country electron configuration and orbital diagram for a selection of atoms in the first and second periods of the periodic tabular array. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of electrons. We start with a single hydrogen atom (atomic number 1), which consists of i proton and one electron. Referring to either Figure \(\PageIndex{three}\) or \(\PageIndex{iv}\), we would await to find the electron in the ones orbital. By convention, the
Post-obit hydrogen is the element of group 0 helium, which has an atomic number of 2. The helium cantlet contains two protons and two electrons. The first electron has the same iv quantum numbers as the hydrogen atom electron (n = 1, l = 0, yard50 = 0, \(m_s=+\dfrac{1}{2}\)
The n = i shell is completely filled in a helium cantlet.
The side by side cantlet is the alkali metallic lithium with an atomic number of three. The first two electrons in lithium fill the ones orbital and have the same sets of four quantum numbers every bit the two electrons in helium. The remaining electron must occupy the orbital of next everyman energy, the twos orbital ( Figure \(\PageIndex{3}\) or \(\PageIndex{four}\) ). Thus, the electron configuration and orbital diagram of lithium are:
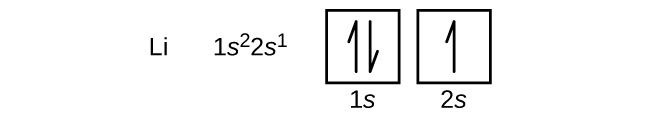
An atom of the element of group ii beryllium, with an atomic number of iv, contains 4 protons in the nucleus and four electrons surrounding the nucleus. The fourth electron fills the remaining space in the 2s orbital.
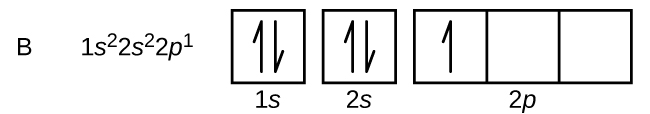
An atom of boron (diminutive number 5) contains five electrons. The n = 1 shell is filled with two electrons and three electrons volition occupy the n = 2 beat out. Because any s subshell tin contain only two electrons, the fifth electron must occupy the next free energy level, which volition be a 2p orbital. There are three degenerate iip orbitals (ml = −one, 0, +1) and the electron can occupy whatsoever one of these p orbitals. When cartoon orbital diagrams, nosotros include empty boxes to draw whatsoever empty orbitals in the aforementioned subshell that we are filling.
Carbon (atomic number half-dozen) has vi electrons. Four of them fill the 1southward and iidue south orbitals. The remaining two electrons occupy the 2p subshell. We now have a option of filling one of the twop orbitals and pairing the electrons or of leaving the electrons unpaired in two different, merely degenerate, p orbitals. The orbitals are filled every bit described by Hund's rule: the lowest-energy configuration for an atom with electrons inside a ready of degenerate orbitals is that having the maximum number of unpaired electrons. Thus, the 2 electrons in the carbon iip orbitals have identical northward, l, and msouthward quantum numbers and differ in their yardfifty quantum number (in accord with the Pauli exclusion principle). The electron configuration and orbital diagram for carbon are:
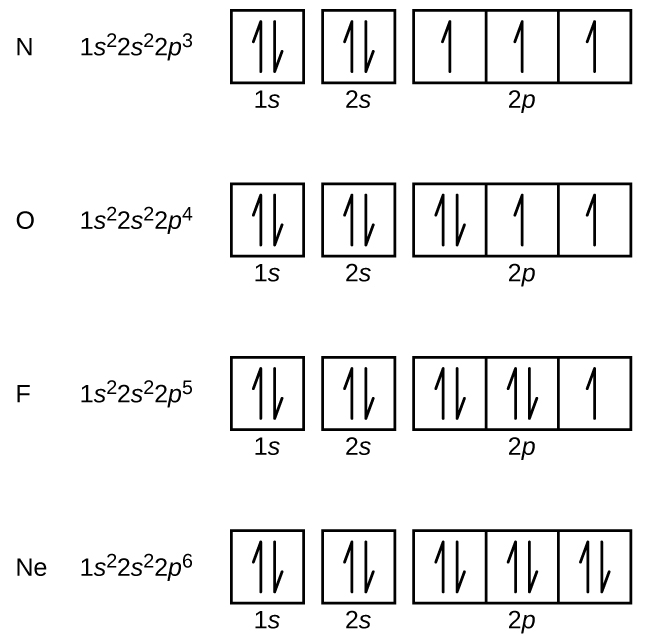
Nitrogen (atomic number 7) fills the 1south and 2s subshells and has one electron in each of the three twop orbitals, in accord with Hund'due south dominion. These iii electrons have unpaired spins. Oxygen (atomic number 8) has a pair of electrons in whatsoever ane of the 2p orbitals (the electrons have reverse spins) and a single electron in each of the other two. Fluorine (atomic number 9) has only i 2p orbital containing an unpaired electron. All of the electrons in the noble gas neon (diminutive number 10) are paired, and all of the orbitals in the northward = 1 and the northward = two shells are filled. The electron configurations and orbital diagrams of these four elements are:
The alkali metal sodium (atomic number 11) has ane more electron than the neon atom. This electron must go into the lowest-energy subshell bachelor, the threes orbital, giving a anesouth 2iis iiiip 6iiisouthward 1 configuration. The electrons occupying the outermost beat out orbital(south) (highest value of n) are chosen valence electrons, and those occupying the inner shell orbitals are chosen core electrons ( Figure \(\PageIndex{5}\)). Since the core electron shells represent to noble gas electron configurations, we tin can abbreviate electron configurations by writing the element of group 0 that matches the cadre electron configuration, forth with the valence electrons in a condensed format. For our sodium example, the symbol [Ne] represents core electrons, (anes two2s 22p half dozen) and our abbreviated or condensed configuration is [Ne]3southward 1.

Figure \(\PageIndex{5}\) : A cadre-abbreviated electron configuration (right) replaces the core electrons with the element of group 0 symbol whose configuration matches the core electron configuration of the other element.
Similarly, the abbreviated configuration of lithium tin can be represented as [He]twos 1, where [He] represents the configuration of the helium atom, which is identical to that of the filled inner shell of lithium. Writing the configurations in this fashion emphasizes the similarity of the configurations of lithium and sodium. Both atoms, which are in the brine metal family unit, have only one electron in a valence due south subshell outside a filled set of inner shells.
\[\ce{Li:[He]}\,2s^1\\ \ce{Na:[Ne]}\,3s^1\]
The element of group ii magnesium (atomic number 12), with its 12 electrons in a [Ne]3s two configuration, is analogous to its family unit member beryllium, [He]2s 2. Both atoms have a filled s subshell outside their filled inner shells. Aluminum (atomic number 13), with 13 electrons and the electron configuration [Ne]3southward 2threep 1, is analogous to its family unit member boron, [He]2southward two2p 1.
The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family unit members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal quantum number of the outer shell of the heavier elements has increased by one to north = 3. Figure \(\PageIndex{6}\) shows the everyman energy, or ground-state, electron configuration for these elements besides as that for atoms of each of the known elements.
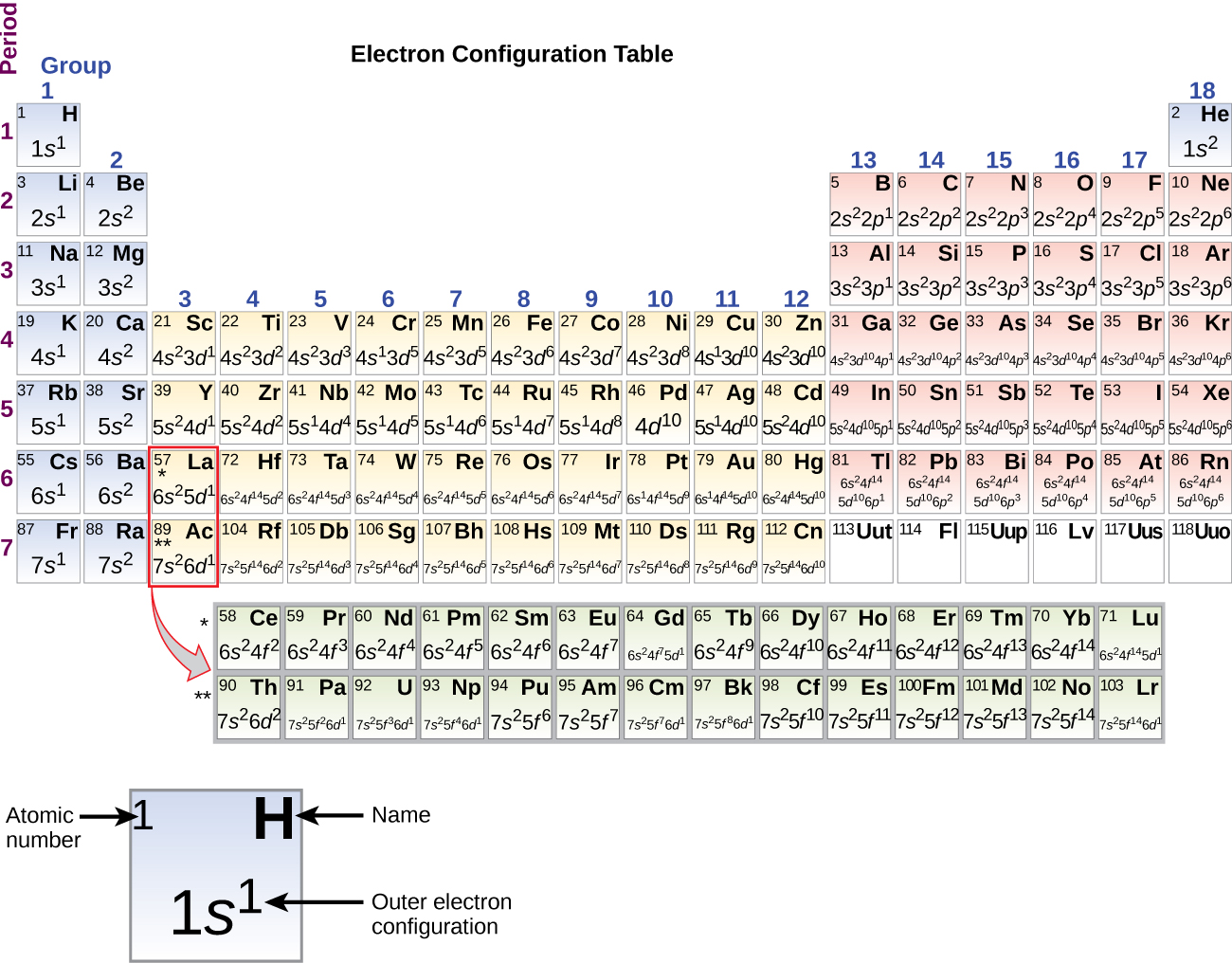
Figure \(\PageIndex{6}\) : This version of the periodic table shows the outer-beat out electron configuration of each chemical element. Annotation that down each group, the configuration is often similar.
When nosotros come to the next element in the periodic table, the alkali metallic potassium (atomic number 19), we might expect that we would begin to add electrons to the 3d subshell. Still, all bachelor chemical and physical evidence indicates that potassium is like lithium and sodium, and that the adjacent electron is not added to the threed level merely is, instead, added to the ivs level (Figure \(\PageIndex{3}\) or \(\PageIndex{4}\)). As discussed previously, the 3d orbital with no radial nodes is higher in energy because it is less penetrating and more shielded from the nucleus than the 4due south, which has iii radial nodes. Thus, potassium has an electron configuration of [Ar]ivs ane. Hence, potassium corresponds to Li and Na in its valence beat configuration. The next electron is added to consummate the 4s subshell and calcium has an electron configuration of [Ar]foursouthward 2. This gives calcium an outer-trounce electron configuration corresponding to that of beryllium and magnesium.
Get-go with the transition metal scandium (diminutive number 21), additional electrons are added successively to the 3d subshell. This subshell is filled to its chapters with 10 electrons (think that for 50 = two [d orbitals], there are twol + ane = 5 values of m50 , meaning that there are 5 d orbitals that take a combined capacity of 10 electrons). The 4p subshell fills next. Note that for iii serial of elements, scandium (Sc) through copper (Cu), yttrium (Y) through silver (Ag), and lutetium (Lu) through gold (Au), a total of 10 d electrons are successively added to the (north – 1) beat out next to the north shell to bring that (northward – i) shell from 8 to 18 electrons. For two series, lanthanum (La) through lutetium (Lu) and actinium (Air-conditioning) through lawrencium (Lr), 14 f electrons (l = 3, iil + 1 = 7 ml values; thus, seven orbitals with a combined capacity of 14 electrons) are successively added to the (n – 2) trounce to bring that shell from eighteen electrons to a total of 32 electrons.
Example \(\PageIndex{1}\): Quantum Numbers and Electron Configurations
What is the electron configuration and orbital diagram for a phosphorus atom? What are the four breakthrough numbers for the concluding electron added?
Solution
The atomic number of phosphorus is fifteen. Thus, a phosphorus atom contains fifteen electrons. The order of filling of the free energy levels is is, twos, 2p, iiis, 3p, ivsouthward, . . . The fifteen electrons of the phosphorus atom will make full to the 3p orbital, which will contain 3 electrons:
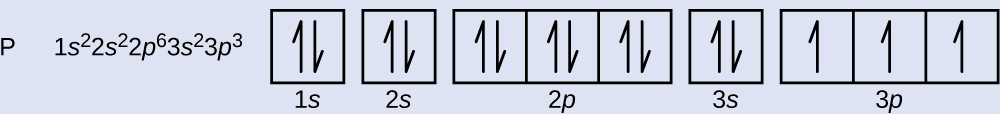
The last electron added is a 3p electron. Therefore, n = three and, for a p-type orbital, l = 1. The one thousand50 value could be –1, 0, or +1. The 3 p orbitals are degenerate, and so whatsoever of these grandl values is correct. For unpaired electrons, convention assigns the value of
Exercise \(\PageIndex{1}\)
Identify the atoms from the electron configurations given:
- [Ar]ivs ii3d v
- [Kr]5s 24d 105p six
- Answer a
-
Mn
- Reply b
-
Xe
The periodic tabular array tin be a powerful tool in predicting the electron configuration of an element. Still, we exercise observe exceptions to the order of filling of orbitals that are shown in Figure \(\PageIndex{three}\) or \(\PageIndex{four}\). For instance, the electron configurations of the transition metals chromium (Cr; atomic number 24) and copper (Cu; atomic number 29), amid others, are not those we would await. In general, such exceptions involve subshells with very like energy, and small-scale effects can pb to changes in the order of filling.
In the case of Cr and Cu, we observe that half-filled and completely filled subshells apparently correspond atmospheric condition of preferred stability. This stability is such that an electron shifts from the ivs into the 3d orbital to gain the extra stability of a half-filled threed subshell (in Cr) or a filled threed subshell (in Cu). Other exceptions likewise occur. For example, niobium (Nb, atomic number 41) is predicted to have the electron configuration [Kr]5s 2ivd 3. Experimentally, we observe that its ground-country electron configuration is actually [Kr]5south 1fourd 4. Nosotros can rationalize this ascertainment by saying that the electron–electron repulsions experienced past pairing the electrons in the fivesouth orbital are larger than the gap in energy between the 5s and 4d orbitals. There is no simple method to predict the exceptions for atoms where the magnitude of the repulsions between electrons is greater than the small-scale differences in energy between subshells.
Electron Configurations and the Periodic Table
Video \(\PageIndex{ane}\) : A trick for writing electron configurations based on the organization of the periodic table.
Equally described earlier, the periodic table arranges atoms based on increasing diminutive number then that elements with the same chemical backdrop recur periodically. When their electron configurations are added to the tabular array (Figure \(\PageIndex{6}\)), we also encounter a periodic recurrence of similar electron configurations in the outer shells of these elements. Because they are in the outer shells of an atom, valence electrons play the about important role in chemic reactions. The outer electrons have the highest free energy of the electrons in an atom and are more easily lost or shared than the core electrons. Valence electrons are likewise the determining factor in some physical properties of the elements.
Elements in any i group (or column) accept the same number of valence electrons; the alkali metals lithium and sodium each have only one valence electron, the alkali metal world metals glucinium and magnesium each take two, and the halogens fluorine and chlorine each have seven valence electrons. The similarity in chemical properties among elements of the same group occurs because they accept the same number of valence electrons. Information technology is the loss, proceeds, or sharing of valence electrons that defines how elements react.
It is important to retrieve that the periodic tabular array was developed on the basis of the chemical behavior of the elements, well before any idea of their atomic structure was available. Now we can sympathise why the periodic tabular array has the organisation it has—the arrangement puts elements whose atoms accept the same number of valence electrons in the same group. This arrangement is emphasized in Figure \(\PageIndex{6}\), which shows in periodic-tabular array grade the electron configuration of the concluding subshell to exist filled by the Aufbau principle. The colored sections of Figure \(\PageIndex{half-dozen}\) show the 3 categories of elements classified by the orbitals being filled: principal grouping, transition, and inner transition elements. These classifications determine which orbitals are counted in the valence shell, or highest energy level orbitals of an atom.
- Principal grouping elements (sometimes called representative elements) are those in which the last electron added enters an s or a p orbital in the outermost shell, shown in bluish and red in Effigy \(\PageIndex{6}\). This category includes all the nonmetallic elements, as well equally many metals and the intermediate semimetallic elements. The valence electrons for principal group elements are those with the highest n level. For example, gallium (Ga, atomic number 31) has the electron configuration [Ar]ivs 2 3d x 4p one , which contains three valence electrons (underlined). The completely filled d orbitals count as core, not valence, electrons.
- Transition elements or transition metals. These are metallic elements in which the final electron added enters a d orbital. The valence electrons (those added after the last noble gas configuration) in these elements include the ns and (n – 1) d electrons. The official IUPAC definition of transition elements specifies those with partially filled d orbitals. Thus, the elements with completely filled orbitals (Zn, Cd, Hg, too as Cu, Ag, and Au in Effigy \(\PageIndex{6}\)) are not technically transition elements. However, the term is ofttimes used to refer to the entire d block (colored yellow in Figure \(\PageIndex{6}\)), and we will adopt this usage in this textbook.
- Inner transition elements are metallic elements in which the terminal electron added occupies an f orbital. They are shown in green in Figure \(\PageIndex{6}\). The valence shells of the inner transition elements consist of the (n – two)f, the (n – ane)d, and the ns subshells. There are two inner transition serial:
- The lanthanide series: lanthanide (La) through lutetium (Lu)
- The actinide serial: actinide (Ac) through lawrencium (Lr)
Lanthanum and actinium, because of their similarities to the other members of the serial, are included and used to proper name the series, even though they are transition metals with no f electrons.
Electron Configurations of Ions
We accept seen that ions are formed when atoms gain or lose electrons. A cation (positively charged ion) forms when i or more electrons are removed from a parent atom. For main group elements, the electrons that were added terminal are the first electrons removed. For transition metals and inner transition metals, however, electrons in the s orbital are easier to remove than the d or f electrons, and so the highest ns electrons are lost, and and then the (north – 1)d or (n – 2)f electrons are removed. An anion (negatively charged ion) forms when one or more electrons are added to a parent atom. The added electrons fill in the order predicted by the Aufbau principle.
Instance \(\PageIndex{2}\): Predicting Electron Configurations of Ions
What is the electron configuration and orbital diagram of:
- Na+
- P3–
- Al2+
- Fe2+
- Sm3+
Solution
Commencement, write out the electron configuration for each parent atom. Nosotros take chosen to show the full, unabbreviated configurations to provide more practice for students who want information technology, merely listing the core-abbreviated electron configurations is also adequate.
Side by side, determine whether an electron is gained or lost. Remember electrons are negatively charged, so ions with a positive accuse have lost an electron. For main group elements, the concluding orbital gains or loses the electron. For transition metals, the last south orbital loses an electron before the d orbitals.
- Na: ones 22s ii2p 63s 1. Sodium cation loses one electron, so Na+: 1s two2s iitwop 6iiisouth 1 = Na+: ones 22due south 2twop six.
- P: 1south 2iis 22p 63s 2iiip 3. Phosphorus trianion gains three electrons, so P3−: 1s 22due south ii2p 6iiisouth two3p 6.
- Al: is 22south 22p 6threes 23p 1. Aluminum dication loses two electrons Al2+: anes 2twos 22p half-dozenthrees 2threep 1 = Alii+: 1southward 22due south 22p 63s 1.
- Iron: 1s 22due south 22p vi3due south ii3p 6foursouth twothreed 6. Iron(II) loses ii electrons and, since information technology is a transition element, they are removed from the 4southward orbital Fetwo+: anes two2southward 22p six3s ii3p 64southward 23d half-dozen = 1southward 22s twoiip 6threesouth 2iiip 6iiid 6.
- Sm: 1s 22s 22p half dozen3southward 23p 64due south 2threed 104p vi5due south 24d xfivep 66s twofourf half-dozen. Samarium trication loses three electrons. The first 2 will be lost from the vis orbital, and the last one is removed from the ivf orbital. Smiii+: 1s 22s 2iip 63southward 2threep 64s 2iiid 104p half-dozenfives 2fourd 105p 66s 24f vi = anesouth 22s 22p 6threes 23p half-dozenfours 23d 104p vi5s two4d 10vp 64f 5.
Exercise \(\PageIndex{2}\)
- Which ion with a +two accuse has the electron configuration 1s 22s ii2p sixthrees 2threep 63d 10ivs two4p sixivd v?
- Which ion with a +3 charge has this configuration?
- Answer a
-
Tc2+
- Reply b
-
Ru3+
Electronic Structures of Cations
When forming a cation, an cantlet of a main group chemical element tends to lose all of its valence electrons, thus assuming the electronic structure of the noble gas that precedes it in the periodic table. For groups 1 (the alkali metals) and two (the alkali metal globe metals), the group numbers are equal to the numbers of valence shell electrons and, consequently, to the charges of the cations formed from atoms of these elements when all valence shell electrons are removed. For case, calcium is a group ii element whose neutral atoms accept 20 electrons and a ground land electron configuration of ones 22s 22p 63due south 2threep vi4s 2. When a Ca atom loses both of its valence electrons, the result is a cation with xviii electrons, a 2+ accuse, and an electron configuration of onesouth 22s ii2p 63south 23p 6. The Ca2+ ion is therefore isoelectronic with the noble gas Ar.
For groups 12–17, the group numbers exceed the number of valence electrons by ten (bookkeeping for the possibility of full d subshells in atoms of elements in the fourth and greater periods). Thus, the accuse of a cation formed past the loss of all valence electrons is equal to the group number minus x. For example, aluminum (in group 13) forms iii+ ions (Al3+).
Exceptions to the expected beliefs involve elements toward the bottom of the groups. In addition to the expected ions Tl3+, Sniv+, Pb4+, and Bifive+, a partial loss of these atoms' valence vanquish electrons tin can besides lead to the germination of Tl+, Sn2+, Atomic number 82ii+, and Bi3+ ions. The formation of these 1+, 2+, and 3+ cations is ascribed to the inert pair effect, which reflects the relatively low energy of the valence s-electron pair for atoms of the heavy elements of groups xiii, xiv, and 15. Mercury (grouping 12) also exhibits an unexpected behavior: it forms a diatomic ion, \(\ce{Hg_2^2+}\)
Transition and inner transition metal elements bear differently than principal grouping elements. Most transition metal cations have ii+ or 3+ charges that consequence from the loss of their outermost s electron(s) first, sometimes followed past the loss of one or 2 d electrons from the adjacent-to-outermost beat. For case, iron (1due south 22s two2p half dozen3due south iiiiip 6iiid 6ivsouthward 2) forms the ion Fe2+ (onedue south 22s 22p half-dozen3south ii3p 6iiid 6) past the loss of the 4s electrons and the ion Fe3+ (1south 22s 2iip 6threes two3p 63d 5) past the loss of the ivs electrons and one of the 3d electrons. Although the d orbitals of the transition elements are—according to the Aufbau principle—the last to fill when building up electron configurations, the outermost due south electrons are the first to be lost when these atoms ionize. When the inner transition metals form ions, they usually have a 3+ charge, resulting from the loss of their outermost s electrons and a d or f electron.
Example \(\PageIndex{3}\): Determining the Electronic Structures of Cations
There are at least fourteen elements categorized as "essential trace elements" for the human trunk. They are called "essential" because they are required for healthy bodily functions, "trace" because they are required merely in small amounts, and "elements" in spite of the fact that they are really ions. Ii of these essential trace elements, chromium and zinc, are required as Cr3+ and Zn2+. Write the electron configurations of these cations.
Solution
First, write the electron configuration for the neutral atoms:
- Zn: [Ar]3d 104s 2
- Cr: [Ar]3d 5ivsouth i
Adjacent, remove electrons from the highest energy orbital. For the transition metals, electrons are removed from the s orbital beginning and then from the d orbital. For the p-block elements, electrons are removed from the p orbitals so from the s orbital. Zinc is a fellow member of group 12, so it should take a accuse of 2+, and thus loses merely the two electrons in its due south orbital. Chromium is a transition element and should lose its s electrons so its d electrons when forming a cation. Thus, we find the following electron configurations of the ions:
- Zn2+: [Ar]3d 10
- Cr3+: [Ar]3d 3
Practise \(\PageIndex{3}\)
Potassium and magnesium are required in our diet. Write the electron configurations of the ions expected from these elements.
- Reply
-
K+: [Ar], Mg2+: [Ne]
Electronic Structures of Anions
Well-nigh monatomic anions form when a neutral nonmetal atom gains plenty electrons to completely fill its outer s and p orbitals, thereby reaching the electron configuration of the next noble gas. Thus, it is simple to determine the accuse on such a negative ion: The charge is equal to the number of electrons that must be gained to fill the south and p orbitals of the parent cantlet. Oxygen, for example, has the electron configuration anes 22s two2p four, whereas the oxygen anion has the electron configuration of the noble gas neon (Ne), 1s iitwos two2p 6. The 2 additional electrons required to make full the valence orbitals give the oxide ion the charge of 2– (O2–).
Instance \(\PageIndex{4}\): Determining the Electronic Structure of Anions
Selenium and iodine are two essential trace elements that form anions. Write the electron configurations of the anions.
Solution
Se2–: [Ar]3d x4southward 2fourp vi
I–: [Kr]fourd ten5s 25p six
Exercise \(\PageIndex{4}\)
Write the electron configurations of a phosphorus atom and its negative ion. Give the charge on the anion.
- Answer
-
P: [Ne]3s 2iiip 3
P3–: [Ne]threes 23p half-dozen
In ionic compounds, electrons are transferred between atoms of unlike elements to form ions. But this is not the simply way that compounds tin can be formed. Atoms can likewise make chemical bonds by sharing electrons between each other. Such bonds are called covalent bonds. Covalent bonds are formed between two atoms when both accept similar tendencies to concenter electrons to themselves (i.e., when both atoms accept identical or fairly similar ionization energies and electron affinities). For example, ii hydrogen atoms bond covalently to class an H2 molecule; each hydrogen atom in the H2 molecule has two electrons stabilizing it, giving each atom the same number of valence electrons equally the element of group 0 He.
Compounds that contain covalent bonds showroom unlike physical properties than ionic compounds. Because the attraction between molecules, which are electrically neutral, is weaker than that between electrically charged ions, covalent compounds generally have much lower melting and boiling points than ionic compounds. In fact, many covalent compounds are liquids or gases at room temperature, and, in their solid states, they are typically much softer than ionic solids. Furthermore, whereas ionic compounds are good conductors of electricity when dissolved in water, most covalent compounds, being electrically neutral, are poor conductors of electricity in any state.
Summary
Video \(\PageIndex{2}\) : An overview of the role of orbitals in electron configurations and how to write electron configurations.
The relative energy of the subshells make up one's mind the order in which atomic orbitals are filled (is, twosouth, 2p, 3s, 3p, ivs, threed, 4p, and so on). Electron configurations and orbital diagrams can be determined by applying the Pauli exclusion principle (no two electrons can take the aforementioned set of iv quantum numbers) and Hund'southward rule (whenever possible, electrons retain unpaired spins in degenerate orbitals).
Electrons in the outermost orbitals, called valence electrons, are responsible for most of the chemical behavior of elements. In the periodic table, elements with analogous valence electron configurations usually occur within the aforementioned group. There are some exceptions to the predicted filling order, particularly when half-filled or completely filled orbitals can be formed. The periodic table tin be divided into three categories based on the orbital in which the terminal electron to be added is placed: main group elements (southward and p orbitals), transition elements (d orbitals), and inner transition elements (f orbitals).
Glossary
- Aufbau principle
- procedure in which the electron configuration of the elements is determined by "building" them in order of atomic numbers, adding ane proton to the nucleus and 1 electron to the proper subshell at a time
- core electron
- electron in an atom that occupies the orbitals of the inner shells
- electron configuration
- electronic structure of an atom in its footing state given as a list of the orbitals occupied by the electrons
- Hund's rule
- every orbital in a subshell is singly occupied with one electron earlier any one orbital is doubly occupied, and all electrons in singly occupied orbitals have the same spin
- orbital diagram
- pictorial representation of the electron configuration showing each orbital as a box and each electron every bit an pointer
- valence electrons
- electrons in the outermost or valence beat out (highest value of northward) of a ground-country atom; determine how an element reacts
- valence crush
- outermost shell of electrons in a footing-state atom; for main grouping elements, the orbitals with the highest due north level (s and p subshells) are in the valence shell, while for transition metals, the highest free energy s and d subshells brand up the valence shell and for inner transition elements, the highest s, d, and f subshells are included
Contributors
-
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors.Textbook content produced past OpenStax Higher is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/85abf193-2bd...a7ac8df6@9.110).
- Adelaide Clark, Oregon Constitute of Engineering
- Crash Form Chemistry: Crash Class is a division of Complexly and videos are free to stream for educational purposes.
Feedback
Accept feedback to requite about this text? Click here.
Found a typo and want extra credit? Click here.
Source: https://chem.libretexts.org/Courses/Oregon_Institute_of_Technology/OIT:_CHE_202_-_General_Chemistry_II/Unit_3:_Periodic_Patterns/3.1:_Electron_Configurations
0 Response to "The Number of Electrons in the Outer Shells of the Members of the Sodium Family"
Post a Comment